Germany Asthma and COPD Therapeutics Market Analysis
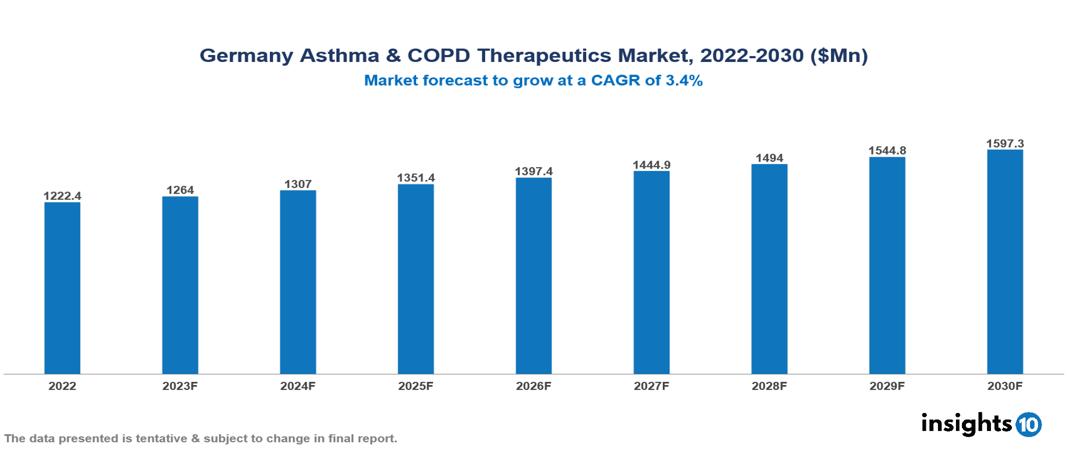
The Germany Asthma and COPD Therapeutics Market was valued at US $1.222 Bn in 2022, and is predicted to grow at (CAGR) of 3.40% from 2023 to 2030, to US $1.597 Bn by 2030. The key drivers of this industry include the rise in the prevalence of COPD and asthma, a focus on quality of life, supportive regulations, and others. The industry is primarily dominated by players such as, GlaxoSmithKline, Teva, Pfizer, Novartis, Sulfateq, AstraZeneca, and Boehringer Ingelheim, among others.
Buy Now

Germany Asthma and COPD Therapeutics Market Analysis: Executive Summary
The Germany Asthma and COPD Therapeutics Market is at around US $1.22 Bn in 2022 and is projected to reach US $1.6 Bn in 2030, exhibiting a CAGR of 3.40% during the forecast period.
Asthma and Chronic Obstructive Pulmonary Disease (COPD) are chronic respiratory disorders that damage the airways and cause difficulty in breathing. Asthma is characterized by inflammation and constriction of the airways, which are frequently induced by allergens, irritants, or exercise. Common symptoms include wheezing, shortness of breath, tightness in the chest, and coughing, which are associated with risk factors such as a family history of respiratory infections. COPD includes chronic bronchitis and emphysema, which cause congested airways, and smoking has been identified as a key risk factor. Identifiable COPD symptoms include a persistent cough, increased mucus production, and exhaustion. Bronchodilators are used to relieve airway tightness in Asthma, and anti-inflammatory medicines are used to treat inflammation in COPD. Medications from firms like GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim are widely recommended.
COPD affects about 12% of the population, and the prevalence of asthma ranges from approximately 7% to 12% among both adults and children in France. These statistics are closely linked to increased levels of smoking, air pollution, and other environmental factors in the population. The primary factors driving the market are the surge in the prevalence of Asthma and COPD, technological advancements, a favorable regulatory landscape, and others. However, the high costs of advanced treatment, underdiagnosis, and increasing generic medications are a few factors that limit the market's potential.
Market Dynamics
Market Growth Drivers
Rising disease prevalence: The estimated prevalence of COPD in Germany is around 12% for the population over 40 years of age which translates to roughly 7 million individuals. Subsequently, the prevalence of asthma is approximately 7% in adults and 12% in children, with more than 1 million children currently suffering from asthma in Germany. With the aging of Germany's population, it is anticipated that the occurrence of both COPD and asthma will rise, attributed to age-related alterations in lung function and heightened vulnerability to respiratory conditions. This translates into a huge patient pool that requires regular treatment, leading to market growth.
Focus on quality of life: While current treatments effectively manage symptoms, there is a continuous need for novel medications that handle disease progression, prevent poor prognosis, and provide long-term management. Patients seek solutions that reduce limitations and improve their overall well-being, fueling the demand for convenient and effective treatment options.
Technological advancements: Exploration into new drug targets, formulations, and delivery systems such as inhalers and nebulizers offers the potential for more efficient and patient-friendly treatment alternatives. The integration of AI applications in diagnostics, treatment selection, and drug development has the capacity to expedite innovation and customize healthcare delivery in Germany.
Supportive regulatory landscape: Healthcare policies in Germany prioritize enhancing access to innovative treatments and early diagnosis, contributing to the positive impact on the market for Asthma and COPD therapeutics. Regulatory reforms are designed to streamline the approval process for new and effective medications, thereby promoting market growth.
Market Restraints
High Cost of Treatment:
Effective management of both COPD and asthma necessitates the long-term use of medications, which constitutes a significant financial burden. The elevated cost of treatment can present a barrier for patients, especially those with limited financial means. A recent estimate suggested the direct medical costs of COPD in Germany at approximately $8 billion, demonstrating the substantial financial strain on both patients and healthcare systems.
Underdiagnosis and misdiagnosis: Even with a significant occurrence of COPD in Germany, numerous cases go undetected or are misdiagnosed. Estimates indicate that only 20–25% of individuals with COPD receive a proper diagnosis. This delay in initiating appropriate treatment can mediate disease progression, contributing to increased long-term healthcare costs.
Rise of generic medications: The growing presence of generic and biosimilar drugs may exert downward pressure on the prices of branded medications, influencing the overall revenue of the market. While this is cost-effective for patients, it also has the potential to impact the profitability of pharmaceutical companies that invest in the research and development of new therapies.
Notable Updates
July 2023, Lupin Pharmaceutical Company introduced a pressurized metered-dose inhaler called Luforbec in Germany. This inhaler combines a corticosteroid and a long-acting beta2-agonist and is designed for the treatment of both asthma and chronic obstructive pulmonary disease (COPD).
Healthcare Policies and Regulatory Landscape
The regulatory landscape governing healthcare in Germany is influenced by directives, standards, and safety regulations issued both at the national level and by the European Union (EU). Oversight of medical devices is the responsibility of the Federal Institute for Drugs and Medical Devices (BfArM), which enforces the Medical Devices Act and the Medical Devices Regulation in alignment with EU directives. Germany's healthcare system is characterized by a decentralized decision-making structure involving the federal government, state laws, and regional associations of sickness funds. The Federal Joint Committee plays a pivotal role in determining the covered services of sickness funds, relying on evidence from comparative effectiveness reviews and health assessments.
Companies aiming for healthcare licensure in Germany must undergo a thorough registration process overseen by the BfArM, facilitated through the electronic portal DIMDI. For new entrants to the German market, a comprehensive understanding of the regulatory environment is essential to ensuring compliance with the relevant regulations.
Competitive Landscape
Key Players
- GlaxoSmithKline
- AstraZeneca
- Boehringer-Ingelheim Pharmaceuticals
- Novartis
- Merck & Co
- Abbott Laboratories
- Merck
- Teva Pharmaceuticals
- Pfizer
- Chiesi Pharmaceuticals
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Asthma and COPD Therapeutics Market Segmentation
By Disease Type
- Asthma
- COPD
By Medication Class
- Combination drugs
- Short Acting Beta Agonists (SABA)
- Long Acting Beta Agonists (LABA)
- Leukotriene Antagonists (LTA)
- Anticholinergics
- Others
By Delivery Device
- Metered-dose inhalers (MDI)
- Dry Powder inhalers (DPI)
- Nebulizers
By Route of Administration
- Inhaled
- Oral
- Others
By End User
- Asthma Patients
- COPD Patients
By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.