Vietnam Oncology Clinical Trials Market Analysis
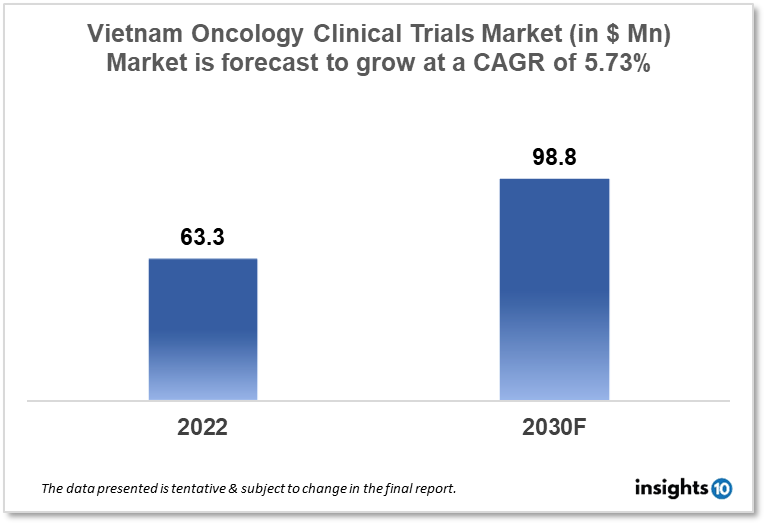
Vietnam's oncology clinical trials market is projected to grow from $63.3 Mn in 2022 to $98.8 Mn by 2030, registering a CAGR of 5.73% during the forecast period of 2022-30. The market will be driven by the big and diversified patient population available for clinical trials and a well-established clinical trial regulatory structure. The market is segmented by phase, by study design & by indication. Some of the major players include AstraZeneca PLC, Novartis AG & Hau Giang Pharmaceutical Joint Stock Company.
Buy Now

Vietnam Oncology Clinical Trials Market Executive Summary
Vietnam's oncology clinical trials market is projected to grow from $63.3 Mn in 2022 to $98.8 Mn by 2030, registering a CAGR of 5.73% during the forecast period of 2022-30. With a population of about 98 Mn people and a life expectancy of over 76 years, Vietnam has a rather significant market size. Roughly 30% of the Vietnamese population, or 30 Mn people, can afford rather costly Western treatment. Vietnam's pharmaceutical business is one of the fastest growing in the area, owing to increased economic development, rising per capita income, and an aging population. Each year, there are 183,000 instances of cancer and 123 thousand fatalities in Vietnam. In Vietnam, the five major cancer types were liver (14.5%), lung (14.4%), breast (11.8%), gastric (9.8%), and colorectal (9%). According to GLOBOCAN, there are now 354,000 cancer patients in the nation.
In addition to hospitals and research institutes such as National Cancer Hospital, Hanoi Medical University, Ho Chi Minh City Oncology Hospital & Bach Mai Hospital, there are several private hospitals and cancer centers in Vietnam that are also engaged in oncology clinical trials. Moreover, In September 2022, K Hospital and Novartis Vietnam signed a Memorandum of Understanding for a collaboration to improve cancer treatment in Vietnam. In march 2023, Gene Solutions, which was founded in Vietnam, designed a multimodal liquid biopsy-based test called Screening for the Presence of Tumour by Methylation and Size (SPOT-MAS) to identify the five most frequent cancer types (liver, breast, colorectal, gastric, and lung cancer). With a big and diversified patient population, a supportive regulatory environment, and an increasing number of qualified clinical researchers and research institutes, Vietnam is an attractive location for oncology clinical trials. Nonetheless, the lack of sophisticated technology and infrastructure remains a barrier that must be overcome in order to attract more sponsors to perform oncology clinical trials in Vietnam.
Market Dynamics
Market Growth Drivers
Many variables contribute to Vietnam's attractiveness as a site for oncology clinical trials. First and foremost, Vietnam has a big and diversified patient population, which is vital for clinical trials, particularly in cancer, where patient selection is critical. Second, Vietnam has a well-established clinical trial regulatory structure, with the Ministry of Health (MoH) in charge of regulating and authorizing clinical studies in the nation.
The government is also aggressively pushing clinical research, particularly oncology trials, and has developed many cancer treatment and research centers of excellence. Moreover, Vietnam has an increasing number of skilled oncologists, clinical researchers, and research institutes that are well-equipped to conduct clinical trials.
Market Restraints
Several growth constraints must be addressed in order to accelerate the expansion of oncology clinical trials in Vietnam. Some of the significant restraints that can affect the expansion and growth of clinical trials in oncology include the limited availability of modern technology and facilities, a scarcity of experienced clinical trial investigators, regulatory challenges, limited patient access and awareness, and funding constraints.
Competitive Landscape
Key Players
- AstraZeneca PLC
- Novartis AG
- FHoffmann-La Roche Ltd
- Pfizer Inc.
- Merck & Co Inc.
- Bristol-Myers Squibb
- Hau Giang Pharmaceutical Joint Stock Company (VNM)
- Vingroup Joint Stock Company (VNM)
Notable Insights
- March 2023, Lotus Pharmaceuticals of Taiwan has won permission in Vietnam for its cancer medicine vinorelbine soft gel in two strengths - 20mg and 80mg - to compete with the branded version Navelbine, developed by French manufacturer Pierre Fabre
- April 2022, Artemis DNA, Inc., located in the United States, will launch its first international facility in Ho Chi Minh City, Vietnam
- March 2022, Roche Pharma Vietnam and the HCM City Oncology Hospital established an agreement to boost the hospital's medical staff's professional ability and to expand collaboration with local and international cancer organizations and specialists
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Clinical Trials Regulation in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
6. Methodology and Scope
Oncology Clinical Trials Market Segmentation
By Phase (Revenue, USD Billion):
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design Outlook (Revenue, USD Billion):
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle regeneration
- Others
By Indication Outlook (Revenue, USD Billion):
- Interventional
- Observational
- Expanded Access
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































