US Advanced Therapy Medicinal Products (ATMPs) Market Analysis
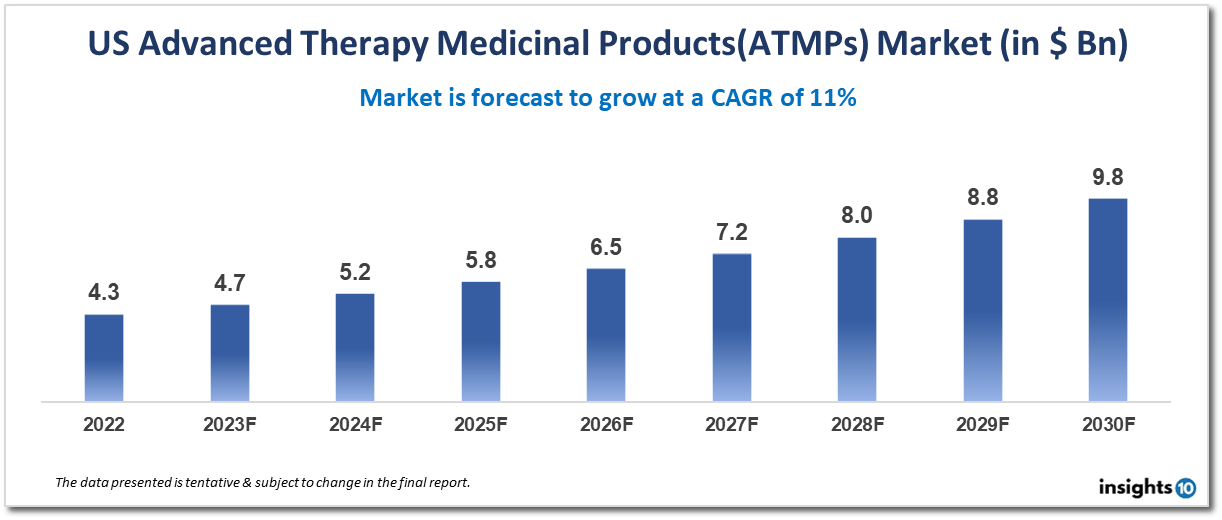
The US Advanced Therapy Medicinal Products (ATMPs) market is projected to grow from $4.3 Bn in 2022 to $9.8 Bn by 2030, registering a CAGR of 11% during the forecast period of 2022-30. One of the key drivers of growth in the US ATMP market is the increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases. These diseases often require long-term treatment, and ATMPs offer a promising new approach to treating them. Additionally, advances in gene editing technologies such as CRISPR/Cas9 have increased the potential for ATMPs to cure genetic disorders. Some of the major players are Novartis International AG, Gilead Sciences, Inc., Bluebird Bio, Inc., Spark Therapeutics, Inc., and Kite Pharma, Inc.
Buy Now

US Advanced Therapy Medicinal Products (ATMPs) Market Executive Summary
It is anticipated that the US Advanced Therapy Medicinal Products (ATMPs) market will increase from $4.3 Bn in 2022 to $9.8 Bn by 2030, recording a CAGR of 11% over the forecast period of 2022-30.
Innovative therapies known as advanced therapy medicinal products (ATMPs) employ living cells, tissues, or genes to treat or cure illness. Due to the fact that they provide a fresh approach to treating complicated and life-threatening illnesses, these treatments are gaining popularity in the US. One of the major markets for ATMPs in the world, the US market is anticipated to expand dramatically over the coming few years.
The rising incidence of chronic illnesses including cancer, diabetes, and cardiovascular diseases is one of the main factors boosting the US ATMPs market. ATMPs provide a promising novel therapeutic strategy for the treatment of many disorders, which frequently require long-term care. Likewise, the potential for ATMPs to treat genetic abnormalities has expanded as a result of developments in gene editing tools like CRISPR/Cas9.
By establishing a regulatory environment that promotes the development of these treatments, the US Food and Drug Administration (FDA) has also significantly contributed to the expansion of the ATMPs industry. The FDA has expedited the approval procedure for these treatments and created a specialized office to supervise the creation and approval of ATMPs.
The US ATMPs market is divided into 3 product categories:
- Tissue engineering
- Cell therapy, and
- Gene therapy
The largest category is gene therapy, which has the ability to treat genetic illnesses by changing or replacing defective genes. Cell therapy, which uses live cells to replace or repair damaged tissues, is another important subsegment.
Notwithstanding the potential advantages of ATMPs, the US market faces a number of difficulties. Several of these medicines require specific manufacturing procedures, and their development is frequently time-consuming and costly. Access to these treatments may also be hampered by their high cost, and certain ATMPs' long-term effectiveness and safety have been questioned.
As these novel medicines provide a fresh approach to treating complicated and life-threatening illnesses, the US ATMPs market is anticipated to expand significantly over the next years. To guarantee the long-term viability of these medicines, the industry will need to solve a number of issues.
Market Dynamics
Market Growth Drivers
- Chronic diseases are becoming more common: One of the main factors propelling the market for ATMPs is the increased incidence of chronic illnesses including cancer, diabetes, and cardiovascular conditions. A potential new method of treating these disorders is provided by ATMPs.
- Improvements in gene editing technologies: The market is expanding as a result of the greater potential for ATMPs to treat genetic illnesses brought about by advances in gene editing technologies like CRISPR/Cas9
- Support from the FDA: The FDA has created a legal framework that promotes the creation of ATMPs, which is fueling the market expansion
- Rising R&D expenditures: The market is anticipated to rise as a result of rising R&D expenditures on ATMPs
Competitive Landscape
Key Players
- Novartis International AG
- Gilead Sciences, Inc.
- Bluebird Bio, Inc.
- Spark Therapeutics, Inc.
- Kite Pharma, Inc.
- Celgene Corporation
- Editas Medicine, Inc.
- Sangamo Therapeutics, Inc.
- Intellia Therapeutics, Inc.
- CRISPR Therapeutics AG
These companies are among the leading developers and manufacturers of advanced therapy medicinal products in the United States. They are involved in the development of innovative treatments for a variety of diseases and conditions using cutting-edge technologies such as gene therapy, cell therapy, and gene editing. These companies are also investing heavily in research and development to bring new treatments to market and meet the growing demand for advanced therapies
Healthcare Policies and Regulatory Landscape
The American healthcare regulatory and policy environment is complicated and ever-changing. The following are some crucial elements of the healthcare policy and regulatory environment.
- Affordable Care Act (ACA): To increase access to healthcare and lower healthcare costs, the Affordable Care Act, popularly known as Obamacare, was passed in 2010. It contains clauses like the individual mandate, which makes it mandatory for people to obtain health insurance and the creation of health insurance marketplaces.
- Both Medicaid and Medicare Millions of Americans are covered by the government-funded healthcare programs Medicare and Medicaid. Although Medicaid covers low-income individuals and families, Medicare covers the elderly and those with certain impairments.
- FDA guidelines: To assure their efficacy and safety, the FDA oversees pharmaceuticals, medical devices, and other healthcare items. Obtaining FDA clearance might take a while and be expensive, but it is required to launch innovative medications.
- The Health Insurance Portability and Accountability Act (HIPAA), which was passed in 1996, establishes guidelines for safeguarding the confidentiality and privacy of patient health information. Moreover, it has clauses that address patient notification in the case of a data breach.
- Value-based care is a type of healthcare delivery that places an emphasis on enhancing patient outcomes while cutting costs. It entails leveraging data to enhance the delivery of care and matching provider incentives with patient results.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
US Advanced Therapy Medicinal Products (ATMPs) Market Segmentation
By Product Type (Revenue, USD Billion):
- Cell Therapy
- Gene Therapy
- CAR-T Therapy
- Tissue Engineered Product
By Disease Type (Revenue, USD Billion):
- Alzheimer's
- Cystic Fibrosis
- Muscular Dystrophies
- Hemophilia
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacy
- Drug Store
- Retail Store
- Online Pharmacy
Insights10 will provide you with the reports within 10 key parameters which are:
- Market Overview
- Market Growth Drivers & Restraints
- Epidemiology of Disease Type
- Market Segmentation
- Market Share
- Competitive Landscape
- Key Company Profiles
- Healthcare Policies & Regulatory Framework
- Reimbursement Scenario
- Factors Driving Future Growth
Based on our many years of experience, we believe that these are the parameters that are critical to decision-making for business stakeholders. Our focused approach to developing reports focused on 10 key parameters, enabled us to arrive at the name “Insights10”.

Stage I: Market Data Collection
Primary Interviews: We have developed a network of experts, freelancers, and researchers across countries through which we engage with local experts to gather key data points and assumptions about each market. We also engage regularly with some of the best market research agencies such as Atheneum, GuidePoint, GLG, etc. to conduct surveys and interviews, and build intelligence. We have language translators as a part of our team, who between them can cover 30+ languages allowing us to extract better local insights.
Secondary Data Collection: We have developed strong expertise and experience in secondary data collection methods for developing unique data sets and research material. We gather data from multiple reliable sources to maintain a high level of accuracy and consistency. The market data is analyzed and forecasted using appropriate statistical and coherent models. The report offers an overall analysis of the market size, growth, and market share as well as a segment-level analysis of the specific market. Our report includes precise, to-the-point information related to the overall market, competition, growth drivers, challenges, regulatory updates, and competition.
Data Sources: We have access to multiple highly reliable free and subscription data sources. We have many years of experience to understand which sources are more dependable for what and which to prefer for the reliable and latest information. The key sources of information include the following, but are not limited to:

Stage II: Market Data Analysis and Statistical Model
Market Trends: We generally look at macro parameters and micro indicators. The macro parameters include changes in government policies, demand and supply of the market, government intervention programs, and major market share. The micro indicators are GDP growth, market size, market volume, etc. We also understand nuances specific to each country like the US, Canada, India, Germany, etc., and have worked across 60+ countries and hence not only understand global trends but how these differ by country, how payment models, market structure, cultural parameters, etc. differ in each country.
Market Sizing and Analysis: Our expert data analytics team has created various market forecast models by employing the top-down approach i.e. starting with the large overall market and segmenting different areas and the bottom-up approach i.e. starting with population and epidemiology and rolling up based on spend, etc., estimating the size of the market, and distributing among the geographic and/or product segments.
The top-down approach is mainly used for new product forecasting and the bottom-up approach is used for demand estimation of any product for different countries summed up to form the total market. We are able to round off insights and build stronger forecasts because we always do both these methods and triangulate the final numbers.
The study on the market covers the analysis of the leading geographies such as Asia-Pacific, Africa, Europe, Middle East, North America, and Latin America for the period of 2022 to 2030. The qualitative analysis covers the industry landscape and trends, market opportunities, competitive landscape, and policy and regulatory scenario, and the quantitative analysis covers different market estimates and forecasts.
Data Triangulation & Validation:
Data triangulation of various sources and results of the research are carried out by benchmarking with reliable sources such as industry statistics, statistical databases, and company-level averages, etc.
We make sure to finalize the numbers in alignment with the market research. Firstly, our internal experts ensure thorough validation and checking to ensure accurate and precise analysis and then validation is also done using a multiple-data analysis model. Two-level validation is done and entails the finalization of the report scope and the way of representation pattern.
(1).png)
Stage III: Interpretation and Presentation
Analysis & Interpretation: The information gathered is then analyzed and synthesized. The second series of interviews are done if necessary to check and validate. The future opportunities are analyzed by understanding product commercialization and many other factors. It also comprises the analysis of data discrepancies observed across various data sources. Information procured from secondary and primary results is then, interpreted by considering the following parameters: (a partial list)
- Establishing market drivers and trends
- Analyzing the regulatory landscape to understand future growth
- Market Segment based analysis to obtain revenue/volume
- Analyzing current needs and determining penetration to estimate the market
Insights: Our reports deliver actionable insights backed with supporting facts and figures to assist you in achieving exemplary growth. Our in-depth analyses are interspersed with relevant insights and statistics to offer an executive-level view of a given market. The description helps in correlating many minor factors affecting the market and their impact on the different segments within the market.
Data curated from the analysis and interpretation are drawn to portray all in one consolidated report.
Presentation & Reporting: The market research report is presented in different forms such as charts by using a scientific approach for easy understanding. Historic, current, and future analysis is provided for each market in terms of both value and volume. The size of the market is interpreted in the US Dollar value and the respective unit, based on the product, for volume consumption.
The foreign exchange rates are calculated on the respective dates and for the respective regions covered in the study.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
This report addresses
- Intelligent insights to take informed business decisions
- Qualitative, acute and result oriented market analysis
- Market size and forecasts from 2022 to 2030
- Opportunities for expansion and in-depth market analysis
- Segmentation and regional revenue forecasts
- Analysis of the market share and competitive landscape
- Strategic Recommendations to chart future course of action
- Comprehensive Market Research Report in PDF and PPT formats
Need more?
- Ask our analyst how this study was put together to learn more
- Discuss additional requirements as part of the free customisation
- Add more countries or regions to the scope
- Get answers to specific business questions
- Develop the business case to launch the product
- Find out how this report may influence your business revenue


