UK Exosome Research Market Analysis
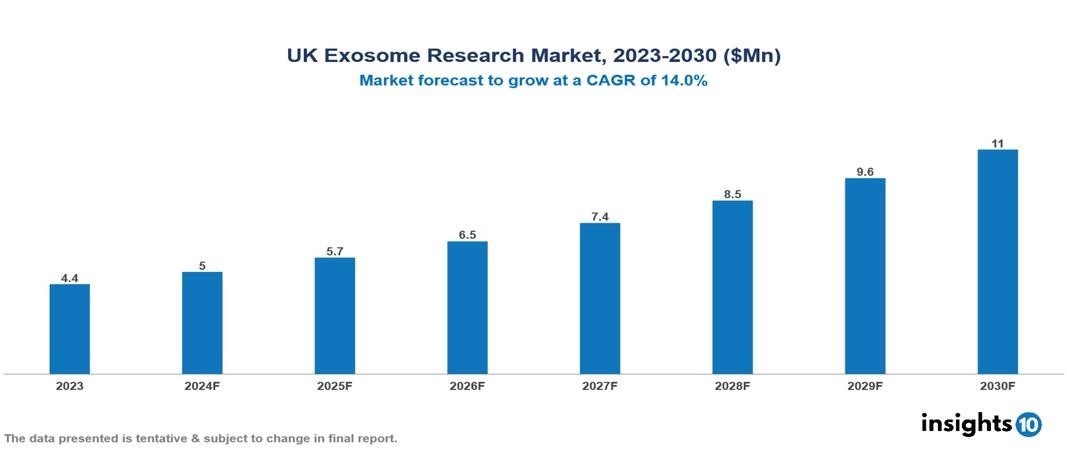
The UK Exosome Research Market was valued at $4.4 Mn in 2023 and is projected to grow at a CAGR of 14% from 2023 to 2023, to $11 Mn by 2030. The key drivers of this industry are increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, increasing advanced applications of exosomes. The industry is primarily dominated by players such as AMS Biotechnology, Evox Therapeutics, Thermo Fisher Scientific among others.
Buy Now

UK Exosome Research Market Executive Summary
The UK Exosome Research Market is at around $4.4 Mn in 2023 and is projected to reach $11 Mn in 2030, exhibiting a CAGR of 14% during the forecast period 2023-2030.
Exosomes are nano-sized extracellular vesicles that play a crucial role in intercellular communication, transporting molecules like proteins, lipids, and nucleic acids between cells. This unique capability has sparked significant interest in the exosome market across various fields. In therapeutics, exosomes show promise as natural drug delivery vehicles, potentially improving treatment specificity and reducing side effects. Their molecular composition also makes them valuable biomarkers for early disease detection and monitoring in diagnostics. The cosmeceutical industry is exploring exosomes, particularly those derived from stem cells, for skin rejuvenation and anti-aging products. In research, exosomes serve as important tools for studying intercellular communication and disease mechanisms, aiding in the development of new therapeutic strategies.
Exosomes offer several advantages over traditional therapies. They function as cell-free alternatives for treating various conditions, capable of delivering therapeutic cargo without triggering immune rejection. Their high biocompatibility and potential for long-term circulation make them suitable for delivering a variety of proteins, chemicals, and nucleic acids throughout the body.
Particularly noteworthy is the potential of exosomes in treating respiratory viral diseases like COVID-19. Stem cell-derived exosomes harness the anti-inflammatory and regenerative abilities of their parent cells, making them promising candidates for engineered treatments. Additionally, exosomes have shown therapeutic properties against various organ injuries and conditions affecting the heart, kidney, liver, and lungs. The versatility and potential of exosomes across multiple medical fields are driving growing interest in the exosome market. As research progresses, exosomes are increasingly seen as a promising platform for developing innovative treatments for a wide range of diseases, potentially revolutionizing approaches to drug delivery and regenerative medicine.
The prevalence rate of cancer in the UK is 39%, there were 166,502 cancer deaths in the UK in 2019, with 88,688 deaths in men and 77,814 deaths in women. The market therefore is driven by significant factors like increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, and increasing advanced applications of exosomes.
Some of the major players operating in the UK Exosome Research Market are AMS Biotechnology, Evox Therapeutics, Thermo Fisher Scientific, Apollo Therapeutics, and Autolus among others.
Market Dynamics
Market Drivers
Strong research and development ecosystem: The UK has a robust research and development ecosystem, with numerous universities, research institutions, and biotechnology companies actively involved in exosome research. This strong scientific foundation drives the development of exosome-based products and technologies.
Government support and funding: The UK government has recognized the potential of exosome research and has provided funding and support for initiatives in this field. For example, the Engineering and Physical Sciences Research Council (EPSRC) and the Biotechnology and Biological Sciences Research Council (BBSRC) have funded exosome-related projects.
Aging population and increasing chronic disease burden: The UK has an aging population, there are about 21 Mn people in UK aged above 50 and over, leading to a higher incidence of age-related diseases like cancer, cardiovascular diseases, and neurodegenerative disorders. Exosome-based diagnostics and therapeutics have shown promise in addressing these conditions, driving market demand.
Market Restraints
Regulatory challenges: As with other countries, the exosome market in the UK faces regulatory challenges due to its novelty. Navigating the regulatory landscape and obtaining approvals for exosome-based products can be a complex and time-consuming process.
High development and production costs: The development and production of exosome-based products can be costly, requiring significant investments in research, manufacturing facilities, and clinical trials. This high cost can be a barrier, particularly for smaller companies or academic institutions.
Reimbursement and market access: Securing reimbursement and market access for exosome-based products in the UK's National Health Service (NHS) can be challenging, as new and innovative therapies often face scrutiny regarding their cost-effectiveness and clinical benefit.
Regulatory Landscape and Reimbursement Scenario
The Medicines and Healthcare products Regulatory Agency (MHRA) is the UK regulatory body responsible for ensuring the safety, efficacy, and quality of pharmaceutical products. The MHRA regulates medicines, medical devices, and blood components for transfusion in the UK, working closely with other bodies to protect and improve public health. Key responsibilities of the MHRA include ensuring safety and quality, promoting innovation, and prioritizing patient safety through education and monitoring. The UK government has implemented various reimbursement policies to help the healthcare industry grow and satisfy the needs of common citizens. These policies include NHS reimbursement for prescription medicines and medical devices, the Voluntary Scheme for Branded Medicines Pricing, Access and Growth (VPAG) to manage the price of branded medicines, the Accelerated Access Review to speed up access to innovative medicines and medical technologies, and the Early Access to Medicines Scheme to provide earlier access to innovative medicines for patients with serious or life-threatening conditions. These reimbursement policies ensure access to necessary medicines and medical devices while controlling costs, contributing to the growth of the healthcare industry and meeting the needs of the population.
Competitive Landscape
Key Players
Here are some of the major key players in the UK Exosomes Research Market:
- AMS Biotechnology
- Evox Therapeutics
- Thermo Fisher Scientific
- Apollo Therapeutics
- NanoSomix
- Autolus
- Malvern Instruments
- Capricor Therapeutic
- Miltenyi Biotec
- Codiak BioSciences Inc.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
UK Exosomes Research Market Segmentation
By Products
- Instruments
- Software
- Reagents and Kits
By Applications
- Diagnostics
- Therapeutic
By Indication
- Cancer
- Cardiovascular Disease
- Neurogenerative Disease
- Infectious Disease
- Others
By Key End Users
- Hospitals
- Cancer Institutes
- Diagnostic Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.