Italy Rheumatology Drugs Market Analysis
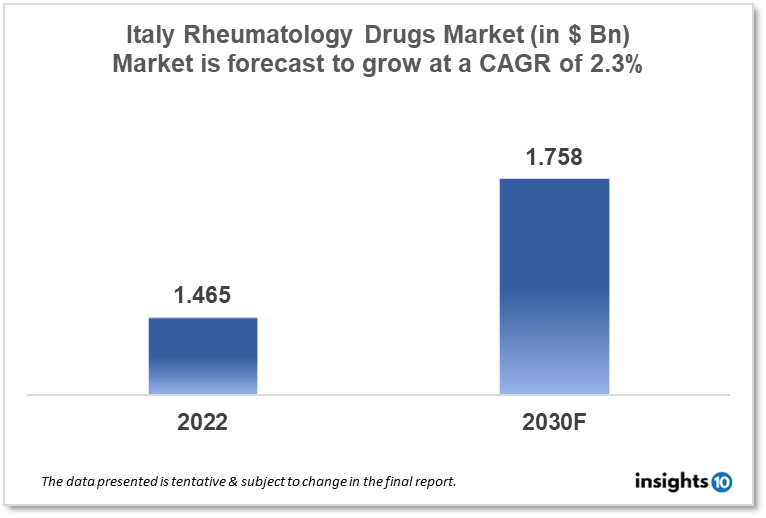
The Italy Rheumatology Drugs Market is projected to grow from $1.465 Bn in 2022 to $1.758 Bn by 2030, registering a CAGR of 2.30% during the forecast period of 2022 - 2030. The major factors driving the growth of the rheumatoid arthritis drugs market are the rise in rheumatoid arthritis prevalence, rise in demand for rheumatoid arthritis drugs, expiration of patents & the entry of biosimilar drugs, rise in geriatric population, rise in adoption of conventional DMARDs, and government initiatives to spread awareness of rheumatoid arthritis symptoms. Some of the key players in the Italy rheumatology drugs market are Sanofi, Roche, and Pfizer
Buy Now

Italy Rheumatology Drugs Market Executive Summary
The Italy Rheumatology Drugs Market is projected to grow from $1.465 Bn in 2022 to $1.758 Bn by 2030, registering a CAGR of 2.30% during the forecast period of 2022 - 2030.
The Italian rheumatology drugs market is a growing market, driven by the increasing prevalence of rheumatic diseases and the growing demand for biologic drugs. Rheumatic diseases, such as rheumatoid arthritis and osteoarthritis, are common in Italy and are becoming increasingly prevalent due to the aging population.
The Italian rheumatology drugs market is characterized by a high degree of competition, with a large number of multinational and local pharmaceutical companies operating in the market. The market is dominated by biological drugs, which are increasingly being used in the treatment of rheumatic diseases due to their efficacy and high level of specificity.
The Italian healthcare system is funded by a combination of public and private funding and is characterized by a high degree of government involvement in the healthcare sector. The government regulates the pricing and reimbursement of drugs and plays a role in the authorization and approval of drugs.
Overall, the Italian rheumatology drugs market is expected to grow in the coming years, driven by the increasing prevalence of rheumatic diseases and the growing demand for biologic drugs. Companies operating in the market are expected to continue to invest in R&D in order to develop more effective rheumatology drugs, which will drive market growth.
Market Dynamics
The major factors driving the growth of the rheumatoid arthritis drugs market are the rise in rheumatoid arthritis prevalence, rise in demand for rheumatoid arthritis drugs, expiration of patents & the entry of biosimilar drugs, rise in geriatric population, rise in adoption of conventional DMARDs, and government initiatives to spread awareness of rheumatoid arthritis symptoms. Other reasons that contribute to the market's expansion include advancements in advanced biologics, an increase in healthcare spending, increased purchasing power, and access to high-quality medications for poor and middle-class families worldwide. However, the major market inhibitors are anticipated to be drug-related adverse effects and the high price of biological DMARD therapy.
Competitive Landscape
Key Players
Some of the key players in the Italy rheumatology drugs market are:
- Roche: Roche is a Swiss pharmaceutical company with a significant presence in Italy. It offers a range of rheumatology drugs, including biologics, such as Actemra and RoActemra, which are used to treat rheumatoid arthritis.
- Pfizer: Pfizer is a global pharmaceutical company with a significant presence in Italy. It offers a range of rheumatology drugs, including biologics, such as Xeljanz, which is used to treat rheumatoid arthritis and psoriatic arthritis.
- Sanofi: Sanofi is a leading pharmaceutical company based in France, with a strong presence in the Italian rheumatology drugs market. It offers a range of rheumatology drugs, including biologics and small-molecule drugs.
- Novartis: Novartis is a Swiss pharmaceutical company with a significant presence in Italy. It offers a range of rheumatology drugs, including biologics, such as Cosentyx, which is used to treat psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.
- AbbVie: AbbVie is a global pharmaceutical company with a significant presence in Italy. It offers a range of rheumatology drugs, including biologics, such as Humira, which is used to treat rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
These key players play a significant role in the Italian rheumatology drugs market, offering a range of effective treatments for rheumatic diseases. The high degree of competition in the market is driving companies to invest in R&D in order to develop more effective rheumatology drugs, which will drive market growth in the coming years.
Healthcare Policies and Regulatory Landscape
The Italian healthcare system is governed by several policies and regulations that have an impact on the rheumatology drugs market. Some of the key policies and regulations are:
- National Health Service (Servizio Sanitario Nazionale, SSN): The National Health Service (SSN) is the public healthcare system in Italy, which provides comprehensive healthcare services to all residents of Italy. The SSN covers the cost of a wide range of healthcare services, including drugs, and is funded by a combination of payroll taxes and general taxation.
- Healthcare Reimbursement: The Italian healthcare reimbursement system is managed by the National Health Service (SSN) and the Ministry of Health. It determines which drugs are eligible for reimbursement and sets the reimbursement rate for each drug.
- Clinical Trials: Clinical trials in Italy are governed by the Italian Medicines Agency (AIFA) and are subject to strict regulations. All clinical trials must be approved by the AIFA and must adhere to Good Clinical Practice (GCP) guidelines.
- Marketing Authorizations: The marketing authorization process for drugs in Italy is managed by the European Medicines Agency (EMA) and the Italian Medicines Agency (AIFA). Drugs must be authorized by the EMA and the AIFA before they can be marketed in Italy.
- Pricing and Reimbursement: The pricing and reimbursement of drugs in Italy are determined by the Ministry of Health, which sets the reimbursement rate for each drug based on its efficacy, safety, and cost-effectiveness.
These policies and regulations play an important role in shaping the Italian rheumatology drugs market, by affecting the availability and accessibility of drugs, as well as the pricing and reimbursement of drugs. Companies operating in the market must comply with these policies and regulations in order to maintain their presence in the market.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Rheumatology Drug Market Segmentation
Drug Class
The disease-modifying anti-rheumatic drugs (DMARDs) are the major revenue contributor. Rise in awareness related to the use of DMARDs, surge in prevalence of rheumatoid arthritis, rise in obese & overweight population across the globe, and increase in adoption of DMARDs as a first-line treatment for rheumatoid arthritis are the key factors that boost the growth of the segment.
- Disease-modifying anti-rheumatic drugs (DMARDs)
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Corticosteroids
- Uric acids
- Others
Route of administration
- Oral
- Parental
Sales channel
Depending on the sales channel, the prescription-based drug segment is the major shareholder in the Itlay rheumatoid drugs market, owing to the advantages offered by prescription-based drugs.
- Prescription-based drugs
- OTC
Insights10 will provide you with the reports within 10 key parameters which are:
- Market Overview
- Market Growth Drivers & Restraints
- Epidemiology of Disease Type
- Market Segmentation
- Market Share
- Competitive Landscape
- Key Company Profiles
- Healthcare Policies & Regulatory Framework
- Reimbursement Scenario
- Factors Driving Future Growth
Based on our many years of experience, we believe that these are the parameters that are critical to decision-making for business stakeholders. Our focused approach to developing reports focused on 10 key parameters, enabled us to arrive at the name “Insights10”.

Stage I: Market Data Collection
Primary Interviews: We have developed a network of experts, freelancers, and researchers across countries through which we engage with local experts to gather key data points and assumptions about each market. We also engage regularly with some of the best market research agencies such as Atheneum, GuidePoint, GLG, etc. to conduct surveys and interviews, and build intelligence. We have language translators as a part of our team, who between them can cover 30+ languages allowing us to extract better local insights.
Secondary Data Collection: We have developed strong expertise and experience in secondary data collection methods for developing unique data sets and research material. We gather data from multiple reliable sources to maintain a high level of accuracy and consistency. The market data is analyzed and forecasted using appropriate statistical and coherent models. The report offers an overall analysis of the market size, growth, and market share as well as a segment-level analysis of the specific market. Our report includes precise, to-the-point information related to the overall market, competition, growth drivers, challenges, regulatory updates, and competition.
Data Sources: We have access to multiple highly reliable free and subscription data sources. We have many years of experience to understand which sources are more dependable for what and which to prefer for the reliable and latest information. The key sources of information include the following, but are not limited to:

Stage II: Market Data Analysis and Statistical Model
Market Trends: We generally look at macro parameters and micro indicators. The macro parameters include changes in government policies, demand and supply of the market, government intervention programs, and major market share. The micro indicators are GDP growth, market size, market volume, etc. We also understand nuances specific to each country like the US, Canada, India, Germany, etc., and have worked across 60+ countries and hence not only understand global trends but how these differ by country, how payment models, market structure, cultural parameters, etc. differ in each country.
Market Sizing and Analysis: Our expert data analytics team has created various market forecast models by employing the top-down approach i.e. starting with the large overall market and segmenting different areas and the bottom-up approach i.e. starting with population and epidemiology and rolling up based on spend, etc., estimating the size of the market, and distributing among the geographic and/or product segments.
The top-down approach is mainly used for new product forecasting and the bottom-up approach is used for demand estimation of any product for different countries summed up to form the total market. We are able to round off insights and build stronger forecasts because we always do both these methods and triangulate the final numbers.
The study on the market covers the analysis of the leading geographies such as Asia-Pacific, Africa, Europe, Middle East, North America, and Latin America for the period of 2022 to 2030. The qualitative analysis covers the industry landscape and trends, market opportunities, competitive landscape, and policy and regulatory scenario, and the quantitative analysis covers different market estimates and forecasts.
Data Triangulation & Validation:
Data triangulation of various sources and results of the research are carried out by benchmarking with reliable sources such as industry statistics, statistical databases, and company-level averages, etc.
We make sure to finalize the numbers in alignment with the market research. Firstly, our internal experts ensure thorough validation and checking to ensure accurate and precise analysis and then validation is also done using a multiple-data analysis model. Two-level validation is done and entails the finalization of the report scope and the way of representation pattern.
(1).png)
Stage III: Interpretation and Presentation
Analysis & Interpretation: The information gathered is then analyzed and synthesized. The second series of interviews are done if necessary to check and validate. The future opportunities are analyzed by understanding product commercialization and many other factors. It also comprises the analysis of data discrepancies observed across various data sources. Information procured from secondary and primary results is then, interpreted by considering the following parameters: (a partial list)
- Establishing market drivers and trends
- Analyzing the regulatory landscape to understand future growth
- Market Segment based analysis to obtain revenue/volume
- Analyzing current needs and determining penetration to estimate the market
Insights: Our reports deliver actionable insights backed with supporting facts and figures to assist you in achieving exemplary growth. Our in-depth analyses are interspersed with relevant insights and statistics to offer an executive-level view of a given market. The description helps in correlating many minor factors affecting the market and their impact on the different segments within the market.
Data curated from the analysis and interpretation are drawn to portray all in one consolidated report.
Presentation & Reporting: The market research report is presented in different forms such as charts by using a scientific approach for easy understanding. Historic, current, and future analysis is provided for each market in terms of both value and volume. The size of the market is interpreted in the US Dollar value and the respective unit, based on the product, for volume consumption.
The foreign exchange rates are calculated on the respective dates and for the respective regions covered in the study.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
This report addresses
- Intelligent insights to take informed business decisions
- Qualitative, acute and result oriented market analysis
- Market size and forecasts from 2022 to 2030
- Opportunities for expansion and in-depth market analysis
- Segmentation and regional revenue forecasts
- Analysis of the market share and competitive landscape
- Strategic Recommendations to chart future course of action
- Comprehensive Market Research Report in PDF and PPT formats
Need more?
- Ask our analyst how this study was put together to learn more
- Discuss additional requirements as part of the free customisation
- Add more countries or regions to the scope
- Get answers to specific business questions
- Develop the business case to launch the product
- Find out how this report may influence your business revenue








































