Global Clinical Trial Patient Recruitment Services Market Analysis
The Global clinical trial patient recruitment services market is projected to grow from $ 0.84 Mn in 2022 to $1.56 Bn by 2030, registering a CAGR of 8% during the forecast period of 2022-30. The Global clinical trial patient recruitment services market is a rapidly growing market that provides services to support the recruitment of patients for clinical trials. Some key players in the global clinical trial patient recruitment services market include IQVIA, Parexel International, PRA Health Sciences, Syneos Health, and WCG Clinical. Other important players include ICON plc, Medpace, Charles River Laboratories, and Premier Research.
Buy Now

Global Clinical Trial Patient Recruitment Services Market Executive Summary
The Global Clinical Trial Patient Recruitment Services Market is projected to grow from $ 0.84 Bn in 2022 to $1.56 Bn by 2030, registering a CAGR of 8% during the forecast period of 2022 - 2030.
The Global Clinical Trial Patient Recruitment Services Market is a rapidly growing market that provides services to support the recruitment of patients for clinical trials. Clinical trial sponsors and research organizations face significant challenges in identifying and recruiting the appropriate patients for their trials. Patient recruitment services offer solutions to these challenges by providing specialized expertise, technologies, and strategies to efficiently identify, engage, and retain patients for clinical trials.
Clinical trial patient recruitment services are specialized services that help researchers and pharmaceutical companies identify and recruit eligible participants for their clinical trials. These services may use a variety of strategies to find potential participants, such as advertising, outreach to medical professionals and patient advocacy groups, and leveraging social media and other digital platforms. The goal is to ensure that trials can recruit enough participants to meet their enrollment goals, while also ensuring that the participants are representative of the broader population that the trial is targeting.
The market is driven by factors such as the increasing demand for clinical trials to support drug development, rising complexities in patient recruitment, and the need for specialized expertise and technologies to support efficient patient recruitment and retention. The market is highly competitive, with numerous companies providing a variety of patient recruitment services. These services may include patient identification, patient engagement, advertising and outreach, patient screening and enrollment, and retention strategies.
The market is expected to continue to grow in the coming years as the need for clinical trials and patient recruitment services increases. However, the market may also face challenges such as regulatory hurdles, data privacy concerns, and the high cost of patient recruitment services. Nonetheless, the increasing adoption of digital technologies and personalized patient engagement strategies are expected to drive the growth of the market in the future.

Use of Electronic Health Records for Recruitment in Clinical Trials
The use of electronic health records (EHRs) in recruitment for clinical trials has the potential to streamline the recruitment process and increase efficiency. By utilizing EHRs, researchers can more easily identify potential study participants and determine their eligibility for a study.
One of the benefits of using EHRs for recruitment is that they can provide a large pool of potential participants who have previously consented to have their medical information used for research purposes. This can reduce the time and effort required to identify eligible participants and gain their consent.
Additionally, EHRs can provide a more complete picture of a patient's health history, including information about previous treatments, diagnoses, and other relevant medical conditions. This information can be used to identify patients who are eligible for a study and to ensure that they meet the study's inclusion criteria.
However, there are also some challenges associated with using EHRs for recruitment. For example, privacy and security concerns must be addressed to ensure that patient's medical information is protected. Additionally, EHR systems are not always interoperable, meaning that data from one system may not be easily transferred to another.
In conclusion, the use of EHRs in recruitment for clinical trials has the potential to improve efficiency and accuracy. However, it is important to carefully consider the benefits and challenges associated with this approach and to ensure that patient's privacy and security are protected.
Pharmacies Step into Trial Space
Large retail pharmacy chains are increasingly acting as clinical trial recruitment sites due to their widespread presence, patient reach, and convenience. These pharmacies have a large customer base that visits them regularly, and many of their customers may be eligible for participation in clinical trials. Additionally, these pharmacies often have established relationships with healthcare providers, which can facilitate referrals for clinical trials.

CVS Health, one of the largest retail pharmacy chains in the United States, has recently entered the clinical trial services market by launching its own clinical trial network called the CVS Health Research Institute in 2021. The goal of this network is to bring clinical trial services to patients in their local communities, leveraging the company's extensive retail pharmacy network and its expertise in managing patient health data.
CVS Health's entry into the clinical trial services market has the potential to expand patient access to clinical trials and improve clinical trial efficiency. By leveraging its existing retail pharmacy network, CVS Health can potentially reach a large number of patients who may be interested in participating in clinical trials. The company can also leverage its health data expertise to support the recruitment and management of clinical trial participants, as well as the collection and analysis of clinical trial data.
CVS Health's approach to clinical trial services is also unique in that it emphasizes the importance of patient-centricity and convenience. For example, the company plans to offer virtual visits and home healthcare services for clinical trial participants, which can make it easier for patients to participate in clinical trials and reduce the burden of travel and other logistical considerations.

Walmart, one of the largest retail corporations in the world, has also recently entered the clinical trial services market by launching its own clinical trial platform called Walmart Health Care Research Institute. The goal of this platform is to provide patients with access to clinical trials and other healthcare services at select Walmart locations.
Walmart's entry into the clinical trial services market has the potential to expand patient access to clinical trials and improve clinical trial efficiency. Similar to CVS Health, Walmart can leverage its existing retail network to reach a large number of potential clinical trial participants. Walmart also plans to offer telemedicine services to support virtual visits with healthcare providers and clinical trial coordinators, which can increase convenience and reduce the burden of travel for clinical trial participants.
Walmart's approach to clinical trial services is also unique in that it emphasizes the importance of reducing healthcare costs and improving health equity. For example, the company plans to offer clinical trial services to underserved and rural communities that may not have access to traditional clinical trial sites or healthcare services.
However, as with any new entrant into the clinical trial services market, there are potential challenges that Walmart may face. For example, there may be concerns about the quality and consistency of data collected from these sites, as well as regulatory and ethical considerations that need to be addressed to ensure the safety and privacy of clinical trial participants.

Walgreens, a pharmacy and retail company that offers a range of healthcare services, including immunizations, health screenings, and medication management also announced the launch of its clinical trial business in June 2022 with the goal of improving access to and participation in sponsor-led drug development research while also redefining the patient experience. In order to overcome obstacles to engaging larger and more varied groups, Walgreens has developed a flexible clinical trial model that integrates the company's extensive foundation of patient information, partner-enabled health and technology capabilities, and in-person and virtual care alternatives.
The launch of Walgreens' clinical trial offerings coincides with recent initiatives by the U.S. Food and Drug Administration to broaden racial and ethnic diversity in clinical trials. Despite the fact that 20% of medications have different responses in different ethnic groups, 75% of clinical trial participants are white, compared to 11% of Hispanic participants and less than 10% of Black and Asian participants.
Market Dynamics
Key Drivers
The global clinical trial patient recruitment services market is driven by several key factors, including:
- Increasing demand for clinical trials: The growing need to develop new treatments for diseases and the rise in the number of clinical trials being conducted globally are driving the demand for patient recruitment services.
- Technological advancements: The use of digital technologies and social media platforms is improving patient recruitment and retention rates, thereby driving the growth of the clinical trial patient recruitment services market.
- Aging population: As the global population ages, the prevalence of chronic diseases increases, leading to a higher demand for clinical trials and patient recruitment services.
- Increasing awareness among patients: The growing awareness among patients about the benefits of clinical trials and the availability of new treatments is driving the demand for patient recruitment services.
- Increasing outsourcing of clinical trial activities: Many pharmaceutical and biotech companies are outsourcing their clinical trial activities, including patient recruitment services, to specialized service providers, which is driving the growth of the market.
Overall, the global clinical trial patient recruitment services market is expected to continue to grow in the coming years, driven by these and other key factors.
Challenges in Clinical Trial Patient Recruitment
- 37% of clinical trials are stopped before testing even begins due to under enrollment
- 85% of trials fail to retain enough patients, and
- 11% are unable to enroll even a single patient
- 30% of patients who do register leave the experiment before it is finished
Clinical trial investigators have long struggled with patient recruiting, which is the leading reason trials are canceled. According to a study review, inadequate recruiting results in the early termination of clinical trials in 19% of cases. Only one-third of US clinical trials with 41 NIH registrations reach their patient recruitment targets, according to one analysis. The same analysis discovered that up to 24% of all NIH-registered trials fall short of obtaining even a partial patient sample.
Because it involves a multitude of circumstances that are outside the control of study sponsors, clinical trial patient recruiting is undoubtedly the most challenging part of pharmaceutical research. Sponsors, investigators, and service providers are nonetheless coming up with inventive new methods to locate and enlist patients despite this loss of control, made feasible by developments in medical technology.
The COVID-19 pandemic has sped up the adoption of these technologies, and now the disruptors and innovators in charge of developing tools for patient recruitment predict that additional technological advancements and improved procedures will remove obstacles to trial participation and hasten research. Here are a few of the novel approaches being used by sponsors and contract research organizations (CROs) to recruit patients.
Competitive Landscape
These clinical trial recruitment companies are some of the best in the business
- IQVIA: With unprecedented patient data and insight, combined with machine learning, IQVIA Direct-to-Patient Recruitment helps eliminate obstacles that add time, cost, and uncertainty to recruitment efforts
- Antidote: Antidote’s mission is to connect sponsors and research sites with informed, engaged patients who are interested in participating in a clinical trial. Services include validated referrals, customized outreach plans, access to a 300+ nonprofit and patient advocacy partner network, and other premium services for trial sponsors
- AutoCruitment: AutoCruitment makes use of more than 1,500 digital channels to connect with patients searching for medical information online. It is also known for its speed and advertises a three-day setup time
- BBK Worldwide: With over 30 years of experience in patient recruitment, BBK Worldwide has a vast library of whitepapers and ebooks to share what they have learned throughout the years
- Clariness: Clariness is an internationally focused recruitment company that has enrolled patients in more than 1,000 trials in 50 countries
- ClinicalConnection: ClinicalConnection works with a database of patients and also features study centers on the website for potential participants to find
- CSSi: As part of their patient recruitment strategy, CSSi connects with local patient organizations to find eligible participants
- Curavit: With decentralized clinical trial execution, Curavit specializes in connecting with patients via telehealth and trial technology platforms
- Elligo Health Research: Elligo Health Research has a time-tested “Elligo Direct” approach that makes it simple for physicians and their patients to take part in clinical trials
- Langland: An advertising agency with branding and clinical trial marketing expertise, Langland is able to blend patient, protocol, disease, and media insights with data and technology to provide recruitment and retention solutions
- MMG: With several decades worth of experience, MMG refers to itself as a team of global recruitment strategists and emphasizes its strategy support capabilities
- Praxis: By offering a range of service options, Praxis allows customers to pick and choose what they need, from digital advertising to community outreach
- Science37: A technology-focused company, Science37 is a virtual clinical trials company that can provide full trial execution or supplemental technology in addition to change management services
- StudyKik: After seeing advertising for the service across Facebook and other platforms, potential participants can sign up for Studykik and be notified when a trial is created for which they may be eligible
- TrialSpark: By partnering with physicians and pharmaceutical companies, TrialSpark reduces the time and expense of clinical trials by providing technology, equipment, and staffing
- TrialX: In addition to patient recruitment services, TrialX also creates mobile apps for trials
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Clinical Trials Regulation in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
6. Methodology and Scope
Global Clinical Trial Patient Recruitment Services Market Segmentation
By Service Type
- Patient Recruitment & Registry Services
- Patient Retention Services
- Others
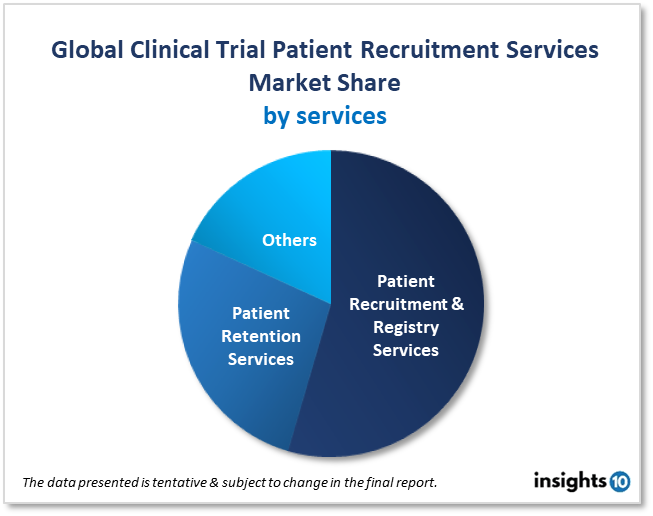
Patient recruitment management offers services for patient registration, patient retention, and other things (analytics, marketing). In 2022, patient recruitment and registry services held a 67.7% market share. It has been shown that 86% of studies do not meet enrollment deadlines, and 30% of phase III trials do not because of enrollment issues.
The most expensive part of clinical trials is patient recruitment, which accounts for 32% of overall costs. Involving patients in the design of new therapies and clinical trials, as well as in the development and post-market processes, helps with patient recruitment and retention and also provides undeniable proof of the worth of medicines in practical applications.
The analysis timeframe is expected to have a significant growth rate of 8.2% for patient retention services. Technology is used in a variety of outreach methods, such as social media, digital marketing, and online health forums. The environment for clinical trials is quickly changing as a result of these activities.
Sponsors, providers for hiring, advertisers, and others can observe behavioral trends when these people are actively engaged online. This is crucial if organizations want to know where to invest money to reach their target patient population when employing online outreach to help with retention.
By Trial Phases
- Phase I
- Phase II
- Phase III
- Phase IV

The market was dominated by the phase III patient recruitment services sector, which required a 57.1% revenue share in 2022. Phase III clinical studies, which are the most costly and involve the most participants, are to blame for this growth. Due to the sample size and the need for sophisticated dosing at an optimum level in the research design, this phase has the highest failure rate. The majority of failures are brought on by noncompliance with safety and efficacy criteria, which result in both human and financial loss.
A growing number of investigational pharmaceuticals are moving forward to phase IV clinical trials, which means that 35.0% of phase III clinical trials are outsourced. This percentage is anticipated to rise.
On the other hand, the phase I segment is predicted to experience a significant growth rate of 9.5% over the course of the projection year. One of the main drivers of the lucrative growth rate of phase I patient recruiting services is the growing number of medicines being investigated for the management and treatment of rare diseases. Additionally, because investigative studies are longer and more likely to result in dropouts, the rate of patients recruited in the first phases is higher and tends to decline throughout the other phases.
By Therapeutic Areas
- Respiratory Diseases
- Pain and Anesthesia
- Oncology
- Central Nervous System
- Cardiovascular
- Endocrine
- Anti-Infective
- Others
The market for clinical trial patient recruiting services is segmented according to therapeutic areas as follows: respiratory diseases, pain and anesthesia, oncology, cardiology, endocrine, respiratory, central nervous system, pain, and others. 13.7% of the market was occupied by the category of pain and anesthesia in 2022. Larger shares of the segment are mostly caused by the expensive clinical research connected to pain and anesthetic, which raises the price of patient recruiting services throughout the illness area.
The market for patient recruiting services connected to oncology is predicted to grow at a profitable CAGR of 9.2% over the forecast period. The segment has experienced rapid development in large part as a result of the recent increase in the number of cancer treatments being developed, which has also increased the need for patient enrolment in such studies. For instance, oncology therapies represented $143 Bn in branded pharmaceutical sales in 2019 or around 20% of all pharmaceutical sales worldwide.
By Age Group
- Child (Birth-17 Years)
- Adult (18 - 64 Years)
- Older Adults (65 Years+)
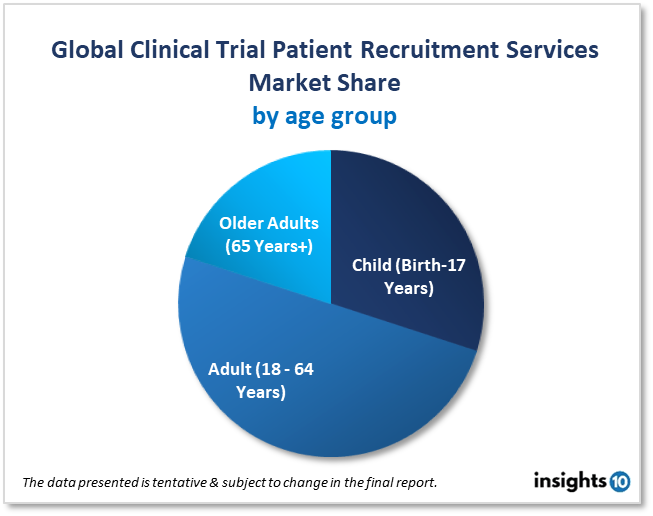
Adult (18–64 Years) was the age group with the biggest market share in 2022, accounting for 49.5%. High shares of the segment are mostly caused by the fact that there were more participants in the age group overall in the clinical investigations that have been done so far. This is because the population in the aforementioned age bracket exhibits greater diversity in studies, and also because it is much safer and healthier for this age group to conduct tests than it is for children or older adults/boomers.
On the other hand, due to the rising prevalence of geriatric illnesses or diseases, the older people (65 Years+) category is expected to see a significant growth rate during the course of the analysis period. The age group has a number of health difficulties that make them a suitable population for clinical investigations, including high blood pressure, nerve damage, vision loss, kidney illness, orthopedic problems, etc.
By Region
- APAC
- Japan
- China
- India
- Australia
- Rest of Asia Pacific
- North America
- US
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Middle East
- Africa
- Latin America

With a 50.4% sales share, North America dominated the global market in 2021. Due to the large number of clinical studies being undertaken in the area, this market is most likely to expand. Government funding for clinical trials and large R&D investments are furthering the market's expansion.
The growth of the North American clinical trial patient recruitment services market has been attributed to the presence of significant CROs providing support services like patient recruitment and multinational pharmaceutical & biopharmaceutical companies making significant investments in clinical research.
With a predicted CAGR of 9.1% during the forecast period, the Asia Pacific clinical trial patient recruitment services market is expected to experience the greatest growth in the region. Because of the low research expenses, simple regulatory compliance, growing patient population, and existence of a few elite clinical institutions serving as sites, the Asia Pacific area has emerged as a hub for conducting clinical trials.
Insights10 will provide you with the reports within 10 key parameters which are:
- Market Overview
- Market Growth Drivers & Restraints
- Epidemiology of Disease Type
- Market Segmentation
- Market Share
- Competitive Landscape
- Key Company Profiles
- Healthcare Policies & Regulatory Framework
- Reimbursement Scenario
- Factors Driving Future Growth
Based on our many years of experience, we believe that these are the parameters that are critical to decision-making for business stakeholders. Our focused approach to developing reports focused on 10 key parameters, enabled us to arrive at the name “Insights10”.

Stage I: Market Data Collection
Primary Interviews: We have developed a network of experts, freelancers, and researchers across countries through which we engage with local experts to gather key data points and assumptions about each market. We also engage regularly with some of the best market research agencies such as Atheneum, GuidePoint, GLG, etc. to conduct surveys and interviews, and build intelligence. We have language translators as a part of our team, who between them can cover 30+ languages allowing us to extract better local insights.
Secondary Data Collection: We have developed strong expertise and experience in secondary data collection methods for developing unique data sets and research material. We gather data from multiple reliable sources to maintain a high level of accuracy and consistency. The market data is analyzed and forecasted using appropriate statistical and coherent models. The report offers an overall analysis of the market size, growth, and market share as well as a segment-level analysis of the specific market. Our report includes precise, to-the-point information related to the overall market, competition, growth drivers, challenges, regulatory updates, and competition.
Data Sources: We have access to multiple highly reliable free and subscription data sources. We have many years of experience to understand which sources are more dependable for what and which to prefer for the reliable and latest information. The key sources of information include the following, but are not limited to:

Stage II: Market Data Analysis and Statistical Model
Market Trends: We generally look at macro parameters and micro indicators. The macro parameters include changes in government policies, demand and supply of the market, government intervention programs, and major market share. The micro indicators are GDP growth, market size, market volume, etc. We also understand nuances specific to each country like the US, Canada, India, Germany, etc., and have worked across 60+ countries and hence not only understand global trends but how these differ by country, how payment models, market structure, cultural parameters, etc. differ in each country.
Market Sizing and Analysis: Our expert data analytics team has created various market forecast models by employing the top-down approach i.e. starting with the large overall market and segmenting different areas and the bottom-up approach i.e. starting with population and epidemiology and rolling up based on spend, etc., estimating the size of the market, and distributing among the geographic and/or product segments.
The top-down approach is mainly used for new product forecasting and the bottom-up approach is used for demand estimation of any product for different countries summed up to form the total market. We are able to round off insights and build stronger forecasts because we always do both these methods and triangulate the final numbers.
The study on the market covers the analysis of the leading geographies such as Asia-Pacific, Africa, Europe, Middle East, North America, and Latin America for the period of 2022 to 2030. The qualitative analysis covers the industry landscape and trends, market opportunities, competitive landscape, and policy and regulatory scenario, and the quantitative analysis covers different market estimates and forecasts.
Data Triangulation & Validation:
Data triangulation of various sources and results of the research are carried out by benchmarking with reliable sources such as industry statistics, statistical databases, and company-level averages, etc.
We make sure to finalize the numbers in alignment with the market research. Firstly, our internal experts ensure thorough validation and checking to ensure accurate and precise analysis and then validation is also done using a multiple-data analysis model. Two-level validation is done and entails the finalization of the report scope and the way of representation pattern.
(1).png)
Stage III: Interpretation and Presentation
Analysis & Interpretation: The information gathered is then analyzed and synthesized. The second series of interviews are done if necessary to check and validate. The future opportunities are analyzed by understanding product commercialization and many other factors. It also comprises the analysis of data discrepancies observed across various data sources. Information procured from secondary and primary results is then, interpreted by considering the following parameters: (a partial list)
- Establishing market drivers and trends
- Analyzing the regulatory landscape to understand future growth
- Market Segment based analysis to obtain revenue/volume
- Analyzing current needs and determining penetration to estimate the market
Insights: Our reports deliver actionable insights backed with supporting facts and figures to assist you in achieving exemplary growth. Our in-depth analyses are interspersed with relevant insights and statistics to offer an executive-level view of a given market. The description helps in correlating many minor factors affecting the market and their impact on the different segments within the market.
Data curated from the analysis and interpretation are drawn to portray all in one consolidated report.
Presentation & Reporting: The market research report is presented in different forms such as charts by using a scientific approach for easy understanding. Historic, current, and future analysis is provided for each market in terms of both value and volume. The size of the market is interpreted in the US Dollar value and the respective unit, based on the product, for volume consumption.
The foreign exchange rates are calculated on the respective dates and for the respective regions covered in the study.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
This report addresses
- Intelligent insights to take informed business decisions
- Qualitative, acute and result oriented market analysis
- Market size and forecasts from 2022 to 2030
- Opportunities for expansion and in-depth market analysis
- Segmentation and regional revenue forecasts
- Analysis of the market share and competitive landscape
- Strategic Recommendations to chart future course of action
- Comprehensive Market Research Report in PDF and PPT formats
Need more?
- Ask our analyst how this study was put together to learn more
- Discuss additional requirements as part of the free customisation
- Add more countries or regions to the scope
- Get answers to specific business questions
- Develop the business case to launch the product
- Find out how this report may influence your business revenue








































